Vaccinations to start in Wales on Tuesday – hope it is ‘turning point’ on ‘long path back to normality’
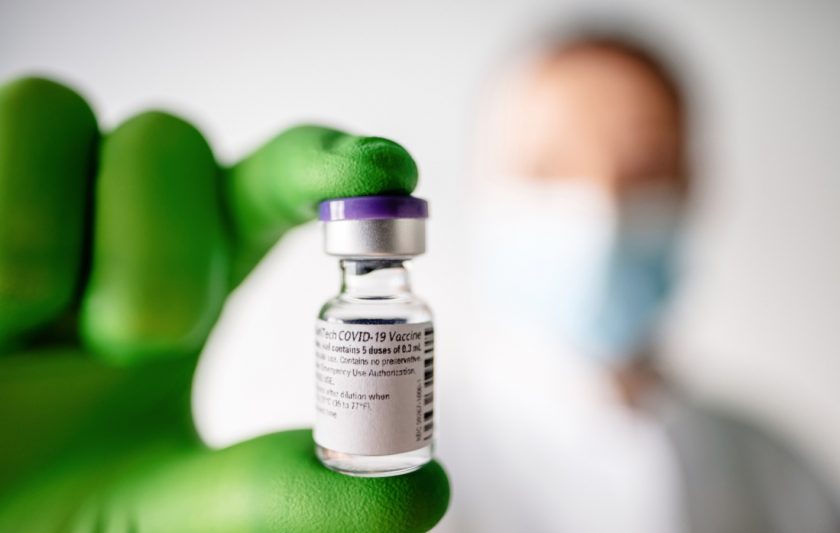
The first phase of the coronavirus vaccination rollout will start in Wales early next week.
It was confirmed that the Pfizer/Biontech MRNA vaccine had become the first to receive Medicines and Healthcare Products Regulatory Agency (MHRA) clearance in the UK.
The MHRA authorised the vaccine as ‘safe and effective’ on the basis of detailed independent expert review of evidence from large scale clinical trials.
40 million doses of the Pfizer/BioNTech vaccine have been secured by the UK Government on behalf of the UK. Wales, like the other UK nations will now begin to receive its share will start deployment, based on advice from the Joint Committee on Vaccination and Immunisation (JCVI).
The vaccine needs to be administered in two doses, 21 days apart.
The first of the 800,000 initial doses of the vaccine started to arrive in the UK on Thursday.
Speaking at Friday’s Welsh Government briefing, First Minister Mark Drakeford said that after many “difficult weeks and months, there is now a glimmer of hope” and that coronavirus vaccinations are set to begin next Tuesday.
Mr Drakeford said: “The first COVID-19 vaccine is ready for use in the UK and we hope that the next one will follow soon.
“Here in Wales, our plans have been thoroughly tested and we expect to receive the first supplies in the next couple of days.
“We have trained staff ready to give the vaccine and I’m pleased to be able to say this afternoon that we are planning to begin vaccinating people from Tuesday of next week.
“We hope of course that this marks a turning point in the pandemic and that it will put us on to what is going to be a long path back to normality.”
A trial rollout of the vaccine, which has to be stored at -70C, has already taken place across Wales ahead of the first phase of the vaccination programme starting next week.
Two specialist sites have been identified as appropriate delivery locations for the vaccine and local health boards will collect the vaccines directly from the two sites.
Details published on the Betsi Cadwaladr University Health Board website explain that on November 26 the end to end logistics for the Pfizer vaccine from ultra-low temperature central storage to receipt by the end user was tested across NHS Wales as part of a “dummy simulation exercise”.
This exercise was a follow up arrangement to an initial test on the 12 November, expanding the logistics to include multiple delivery sites and aligning all the necessary consumables required to undertake a vaccination clinic.
The vaccination process will take place in phases starting with hospital sites and then community settings. People will be sent appointments with details of the location where they will receive the vaccination, dependent on where they are on the schedule and risk.
At the press conference the first minister was asked how confident he was that a vaccine rollout for staff and patients who are eligible will be carried out swiftly.
He said: “Here in Wales, we had a big exercise in the NHS only a week or so ago to test all of those things.
“We are as confident as we can be that when the vaccine arrives and we start vaccinating on Tuesday, and we will be able to deliver it to those frontline staff and those most vulnerable people who we want to get to first.”
Earlier this week Wales’ Chief Medical Officer Dr Frank Atherton moved to allay concerns people may have over the safety of the vaccine and the relatively short timescale it has been developed in.
Speaking on Wednesday, Dr Atherton quoted a population survey that found that 70% of the population will be “be looking to accept a vaccine when it’s available.”
He said: “The most obvious question we’re asked is, is this vaccine safe? And that’s the first and foremost question in any of our minds.
“We take good comfort from the fact that the MHRA has looked very carefully at the data that’s been submitted by in this case Pfizer.
“It’s looked at both the safety and the effectiveness, it’s looked at the trials they’ve run. And it’s concluded that there is sufficient data on safety and effectiveness to allow this to be authorised for use in the UK.
“This has been done in record time in 10 months, so that reflects well on the scientists around the world, not just in the UK, but globally, who invested time and energy in this.
“It reflects the huge investment in research and development from the UK Government and other governments and research agencies.
“It also reflects the fact that organisations of regulatory authorities, like the MHRA, have managed to reduce the paperwork in the bureaucracy while maintaining those critical aspects of safety and effectiveness.”
You can view the full briefing from lunchtime on the below video:
Yn fyw nawr gyda’r Prif Weinidog | Live now with First Minister, Mark Drakeford https://t.co/aMIetu5Z8o
— Welsh Government (@WelshGovernment) December 4, 2020
Spotted something? Got a story? Email: [email protected]
Latest News
