New Pfizer pill approved in UK for people at high risk from Covid
A new oral COVID-19 treatment has been approved for adults in Britain who are at high risk of developing severe infection.
The treatment called Paxlovid has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) after it was found to be safe and effective at reducing the risk of hospitalisation and death in people with mild to moderate COVID-19 infection who are at an increased risk of developing severe disease.
The approval follows a rigorous review of its safety, quality and effectiveness by the UK regulator and expert advice from the UK government’s independent scientific advisory body, the Commission on Human Medicines.
Developed by Pfizer, Paxlovid is an antiviral medicine with a combination of active ingredients, PF-07321332 and ritonavir, that works by inhibiting a protease required for virus replication. This prevents it from multiplying, keeping virus levels low and helping the body to overcome the viral infection. Ritonavir slows the breakdown of the second ingredient PF-07321332 in the body, thereby increasing its effectiveness.
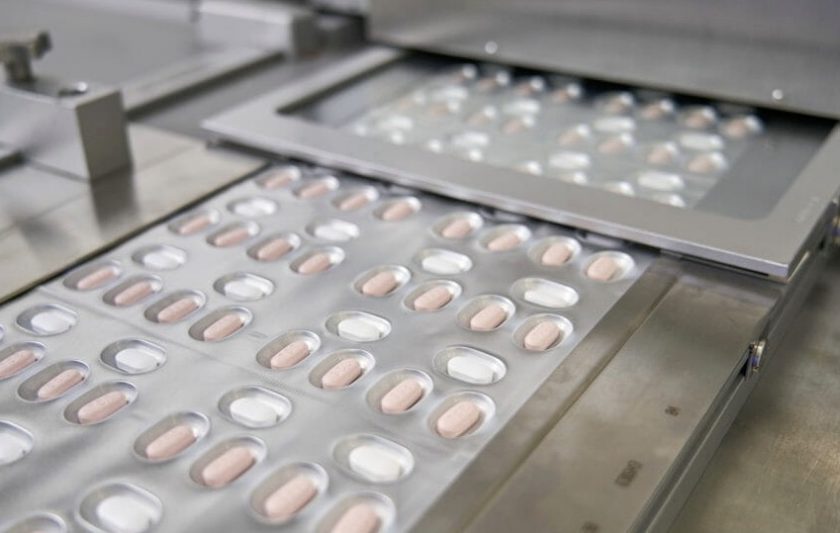
The two active substances of Paxlovid come as separate tablets that are packaged together and taken together, twice a day by mouth for 5 days. PF-07321332 is a new antiviral, meaning that it has not been approved for use before. However, ritonavir has been used alongside some HIV medicines for many years to ‘boost’ their activity, which is similar to what it is doing for PF-07321332.
In a clinical trial in high-risk adults with symptomatic COVID-19 infection, a five day treatment course of Paxlovid reduced the risk of COVID-19 related hospitalisation and death within 28 days by 89% when compared to a placebo group when treatment was started within 3 days of the onset of COVID-19 symptoms.
The number of hospitalisations and deaths were 0.8% (3 out of 389) in the Paxlovid group compared with 7% (27 out of 385) in the placebo group. Similar favourable results were seen in patients when treatment was started within 5 days of the start of symptoms.
Based on the clinical trial data, Paxlovid is most effective when taken during the early stages of infection and so the MHRA recommends its use as soon as possible and within five days of the start of symptoms.
It has been authorised for use in people aged 18 and above who have mild to moderate COVID-19 infection and at least one risk factor for developing severe illness. Such risk factors include obesity, older age (>60 years), diabetes mellitus, or heart disease.
Dr June Raine, MHRA Chief Executive, said:
“Today we have given our regulatory approval for Paxlovid, a COVID-19 treatment found to cut COVID-19 related hospitalisations and deaths by 89% when taken within three days of the start of symptoms.
“We now have a further antiviral medicine for the treatment of COVID-19 that can be taken by mouth rather than administered intravenously. This means it can be administered outside a hospital setting, before COVID-19 has progressed to a severe stage.
“I hope the announcement today gives reassurance to those particularly vulnerable to COVID-19, for whom this treatment has been approved. For these individuals, this treatment could be life-saving.”
[Photo: Pfizer]
Spotted something? Got a story? Email: [email protected]
Latest News
